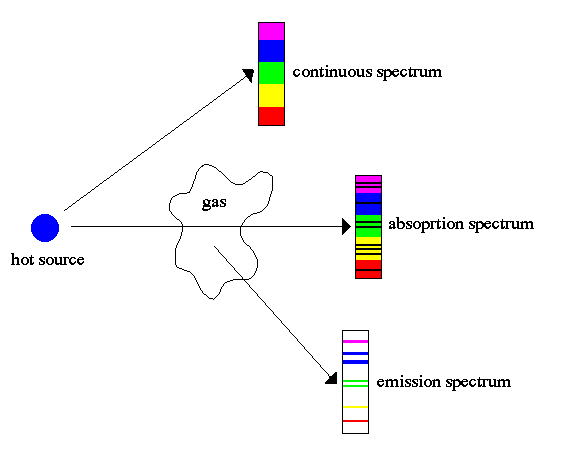
As described in Chapter 3, a blackbody object emits radiation of all wavelengths. However, when the radiation passes through a gas, some of the electrons in the atoms and molecules of the gas absorb some of the energy passing through. The particular wavelengths of energy absorbed are unique to the type of atom or molecule. The radiation emerging from the gas cloud will thus be missing those specific wavelengths, producing a spectrum with dark absorption lines.
The atoms or molecules in the gas then re-emit energy at those same wavelengths. If we can observe this re-emitted energy with little or no back lighting (for example, when we look at clouds of gas in the space between the stars), we will see bright emission lines against a dark background. The emission lines are at the exact frequencies of the absorption lines for a given gas. These phenomena are known as Kirchhoff’s laws of spectral analysis:
1. When a continuous spectrum is viewed through some cool gas, dark spectral lines
(called absorption lines) appear in the continuous spectrum.
1. If the gas is viewed at an angle away from the source of the continuous spectrum, a
pattern of bright spectral lines (called emission lines) is seen against an other-wise
dark background.
Kirchhoff's Law of Spectral Analysis

The same phenomena are at work in the non-visible portions of the spectrum, including the radio range. As the radiation passes through a gas, certain wavelengths are absorbed. Those same wavelengths appear in emission when the gas is observed at an angle with respect to the radiation source.
Why do atoms absorb only electromagnetic energy of a particular wavelength? And why do they emit only energy of these same wavelengths? What follows here is a summarized explanation, but for a more comprehensive one, see Kaufmann’s Universe, pages 90-96.
The answers lie in quantum mechanics. The electrons in an atom may be in a number of allowed energy states. In the atom’s ground state, the electrons are in their lowest energy states. In order to jump to one of a limited number of allowed higher energy levels, the atom must gain a very specific amount of energy. Conversely, when the electron “falls” to a lower energy state, it releases a very specific amount of energy. These discrete packets of energy are called photons.
Thus, each spectral line corresponds to one particular transition between energy states of the atoms of a particular element. An absorption line occurs when an electron jumps from a lower energy state to a higher energy state, extracting the required photon from an outside source of energy such as the continuous spectrum of a hot, glowing object. An emission line is formed when the electron falls back to a lower energy state, releasing a photon.
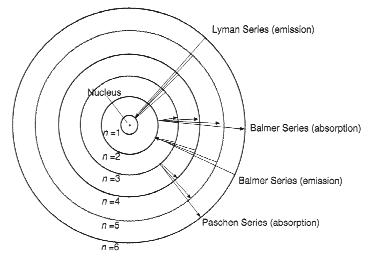
The diagram on the next page demonstrates absorption and emission of photons by an atom using the Neils Bohr model of a hydrogen atom, where the varying energy levels of the electron are represented as varying orbits around the nucleus. (We know that this model is not literally true, but it is useful for describing electron behavior.) The varying series of absorption and emission lines represent different ranges of wavelengths on the continuous spectrum. The Lyman series, for example, includes absorption and emission lines in the ultraviolet part of the spectrum.
Hydrogen Atom
(with allowed electron energy levels n = 1,2,3 etc.)
Emission and absorption lines are also seen when oppositely charged ions recombine to an electrically neutral state. The thus formed neutral atom is highly excited, with electrons transitioning between states, emitting and absorbing photons. The resulting emission and absorp-tion lines are called recombination lines. Some recombination lines occur at relatively low frequencies, well within the radio range, specifically those of carbon ions.
Molecules, as well as atoms, in their gas phase also absorb characteristic narrow frequency bands of radiation passed through them. In the microwave and long wavelength infrared portions of the spectrum, these lines are due to quantized rotational motion of the molecule. The precise fre-quencies of these absorption lines can be used to determine molecular species. This method is valuable for detecting molecules in our atmosphere, in the atmospheres of other planets, and in the interstellar medium. Organic molecules (that is, those containing carbon) have been detected in space in great abundance using molecular spectroscopy. Molecular spectroscopy has become an extremely important area of investigation in radio astronomy.
As will be discussed in Chapter 5, emission and absorption lines in all spectra of extraterrestrial origin may be shifted either toward higher (blue) or lower (red) frequencies, due to a variety of mechanisms.