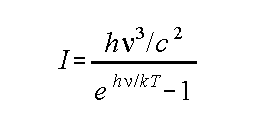
Radio Astronomy Emission Mechanisms
Ronald J. Maddalena
Advanced undergraduate or lower graduate level:
M. Harwit, "Astrophysical Concepts" (1st or 2nd editions).
G. Verschuur and K. Kellerman (eds.), "Galactic and Extra-Galactic Radio Astronomy" (1st and 2nd editions; 1st is at a lower level than 2nd).
G. Verschuur, "Invisible Universe Revealed".
Kraus, "Radio Astronomy" (latest edition).
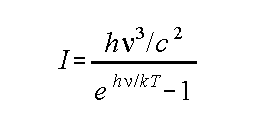
Planck's Law:
h = Plank's constant;
k = Boltzman's constant
T in K, in Hz
INTENSITY is the energy emitted by an object per unit surface area at a certain frequency. It is INTRINSIC to the source -- all observers, no matter where they are in the universe would see the same I(). Units: ergs/cm2/sec/Hz
In radio (approximately):
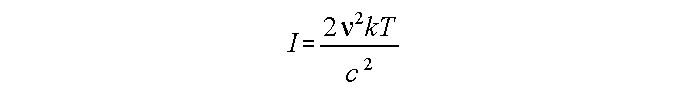
Anything that doesn't follow the "black-body" intensity curve.
I'll only consider two non-thermal methods:
Free-Free
Synchrotron
Whenever a charge particle is accelerated it gives off
radiation.
Electrons moving at relativistic velocities across a magnetic field will spiral around the lines of magnetic force.
Particle experiences an acceleration. In most cases:
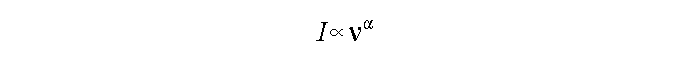
where alpha usually has a value between -0.2 and -1.2
at high frequencies.
Very different from thermal or free-free.
Any mechanism that can produce radiation can also absorb it.
Space does not have an index of refraction equal to one -- electrons in space.
Speed at which light travels = c times the index of refraction.
Index of refraction is different at different frequencies.
Different frequencies will travel at different speeds.
Some frequencies will arrive earlier or later than others.
If an object is moving toward or away from the observer, the radiation spectrum is shifted by approximately:
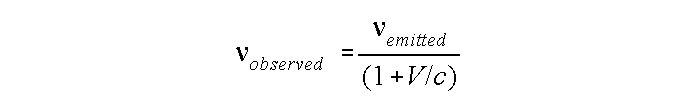
V = velocity of the object toward or away from us. V is < 0 if moving toward us, > 0 if away.
Spectra are shifted (and distorted) if object and observer move toward or away from one another.
Makes no difference whether it is the source or observer who is moving.
The ENERGY of atoms and molecules is quantized.
They lose energy in quantized amounts.
They can lose energy by emitting light.
E = h x frequency so the frequency of the light emitted can only be at certain frequencies.
Note: anything that emits light can also absorb it so atoms and molecules can
absorb light but only if the light have the frequencies that correspond to the
quantized energy.
Atoms can emit or absorb radiation as electrons move from one orbit to another. For hydrogen:
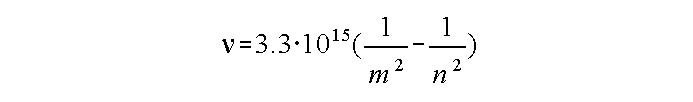
n and m are the 'number' of the electron orbits
If n and m are small numbers, the radiation occurs in or near the visible.
If n and m are large (e.g., m = 176 and n = 177), the radiation will occur at radio frequencies.
Electrons and protons have "spin".
Spin is quantized.
In a H atom, the proton's "spin" may be aligned or anti-aligned with the electron's spin.
A H atom with the spins aligned has more energy than one that is anti-aligned.
An 'aligned' H atom in 11 million years will flip the spin of the electron and emit light at 1420 MHz.
1067 H atoms in the Milky Way ==> about 3x1052 H
atoms per second are emitting at 1420 MHz.
Molecular rotations and vibrations are also quantized.
When a molecule slows its vibration, it might emit at infra-red frequencies.
When a molecule slows its rotation, it might emit at radio frequencies.
Molecules can also produce "Maser" emission -- equivalent to "laser" emission except at radio frequencies and is a natural phenomenon.